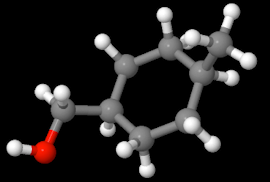
Breaking the name down - the root hexane means the structure has 6 single bonded carbon atoms and the cyclo- prefix means that the structure is a 3D circle rather than a chain.
The prefix 4-methyl means that on the 4th carbon atom of the cyclohexane structure a methyl (CH3) functional group is attached.
And the methanol (the CH2-OH) is the base structure of this compound. It is constructed of a methane (CH4) where one of the hydrogen atoms substituted with a hydroxyl (-OH) - thus methanol. By default this carbon atom is designated "1" since it is the only carbon in the methanol - basically imagine the 4-methylcyclohexane as a functional group hanging off of the methanol.
So, you could write this name as 4-methyl 1-cyclohexane methanol if that helps, and (4-Methylcyclohexyl)methanol is even better (and the real IUPAC name I thought, but it doesn't seem to be showing up in that form many places) - the suffix -yl indicates that a structure is a functional group.
Here is a 3D representation of the structure from http://www.chemspider.com/Chemical-Structure.105625.html
Based on this structure we can tell that this molecule is soluble in water to at least some extent (due to the methanol, which is totally miscible in water). But we don't have a lot more to go on. Most of the opinions floating around out there about the toxicity and nature of this compound are based on what is known about similar compounds and what the structure can tell us. However, fairly small differences in structure can sometimes mean huge differences in toxicity.
Due to the complex nature of IUPAC nomenclature, lots of incorrect information has been going around about the nature of this compound as well. 4-methylcyclohexane methanol is not the same thing as methylcyclohexane, nor is it the same thing as methylcyclohexanol (as was briefly reported on Wikipedia).
Methylcyclohexane is a colorless liquid that is lighter than water (density 0.77 g/cm3 roughly) and fairly insoluble (around 18 ppm at 40°C). The acute oral LD50 for this compound is 2,250 ppm for mice.
If you add this structure to a methanol, that increases its solubility in water relative to methylcyclohexane dramatically, but because this is a pretty large molecule its solubility will still be somewhat lowish - so a portion of this compound will be floating on the water, a portion will be dissolved in the water and some of this will likely bond to the soil as well. According to the Chemical Spider website, one estimate of its solubility in water is 5244.8 ppm in water. Usually compounds like this (hydrocarbons and alcohols) are broken down by bacteria in the environment, but since it is winter, that will slow down the rate of any natural bio-remediation. The octanol-water partition coefficient estimate seems to suggest this compound will not bio-accumulate significantly, but opinions on this seem to be a bit mixed.
As this suggests, unfortunately, it that the expert opinions reported by various news sources are somewhat contradictory in terms of how they expect this compound to behave. There has also been little discussion of what the effects of chronic exposure (small amounts over a long period of time) might be, since it seems rather likely that 4-methylcyclohexane methanol has been leaking from this storage facility for a long time before that 1 inch hole developed, releasing enough of the compound for it to become obvious. How long have people been drinking trace amounts and is it likely to be a carcinogen? a mutagen? a teratogen ? We don't appear to have any solid information on any of that.
According to The Charleston Gazette ...
When state inspectors arrived at the Freedom Industries tank farm late last Thursday morning, they found a 400-square-foot pool of clear liquid had collected outside a white tank marked as number 396.
A 4-foot wide stream of the liquid -- thicker than water, but not as heavy as syrup -- was flowing across the bottom of a containment dike. The flow disappeared right at the joint where the dike's wall connected to its floor.
Freedom Industries had set up one cinder block and used one 50-pound bag of some sort of safety absorbent powder to try to block the chemical flow, state Department of Environmental Protection inspectors say.
"This was a Band-Aid approach," said DEP air quality inspector Mike Kolb. "It was apparent that this was not an event that had just happened."
...
In one enforcement order, DEP officials allege that the company had taken "no spill containment measures" prior to agency staffers arriving at the site and discovering the leak.
"The facility did not give any real attention to containment," Bauerle said.
State and county officials have described the Freedom facility's spill containment dike as full of cracks and holes.
This certainly leads credence to my suspicion that small amounts of 4-methylcyclohexane methanol have been leaking from the site for a long time.
One of the things that seems to have taken many people by surprise is the fact that there isn't much information about 4-methylcyclohexane methanol and its possible impacts on either people or the environment. In reality, this is pretty common. There are tens of thousands of chemicals in common use everyday and very few of them have much of a toxicological workup.
Oh, if you are interested, I have finally found a standard format MSDS (actually it looks like it is following the new SDS format) at
http://i2.cdn.turner.com/cnn/2014/images/01/10/msds.sheet.for.4-methyl-1-cyclohexanemethanol.pdf
Here is the Health Information
CARCINOGENIC EFFECTS: Not available.
MUTAGENIC EFFECTS: Not available.
TERATOGENIC EFFECTS: Not available.
DEVELOPMENTAL TOXICITY: Not available.
Another MSDS is available here
http://online.wsj.com/public/resources/documents/Eastman.pdf
This mixture contains 68-89% 4-methylcyclohexane methanol. It is longer, but still is mostly filled in with "No data available"

No comments:
Post a Comment
Hi! I do read all of the comments and want to let you know that I really appreciate your stopping by and taking the time to leave a note. Work has fallen in on me and I have not had enough time to reply coherently lately so I apologize preemptively but still want to assure you that your comments are valued. I am using comment moderation to avoid using more annoying spam avoidance. Thanks for your patience.